
In skiing, physics is everything. It determines what kind of snow falls, its consistency, and how it changes over time once it has fallen on the ground. One big concept that factors into these processes is evaporative cooling. This article will look at evaporative cooling and why it’s so important.
Essentially, evaporative cooling describes the cooling of an object due to water vapor. For example, say the air temperature is 50ºF, and you and your friends are going swimming. Before you get in, the air feels nippy but not terrible. However, once you get out before you dry off, the air feels much colder. This is due to evaporative cooling, which revolves around the fact that it takes thermal energy to turn water into water vapor.
The same principle is true for falling snow. Moisture in the snow crystal lowers the temperature as it falls, allowing snowflakes to fall even when temperatures are above freezing.
We have a way to measure evaporative cooling: the wet-bulb temperature. This term originates from how it was originally calculated by wrapping a wet towel around the bulb of a thermometer, which allowed the thermometer to measure the temperature of the air with evaporative cooling factored in. Some weather services may give the wet-bulb temperature, but it’s straightforward to calculate on your own if they don’t. You can use a calculator like this one to obtain the wet bulb temperature from temperature and relative humidity.
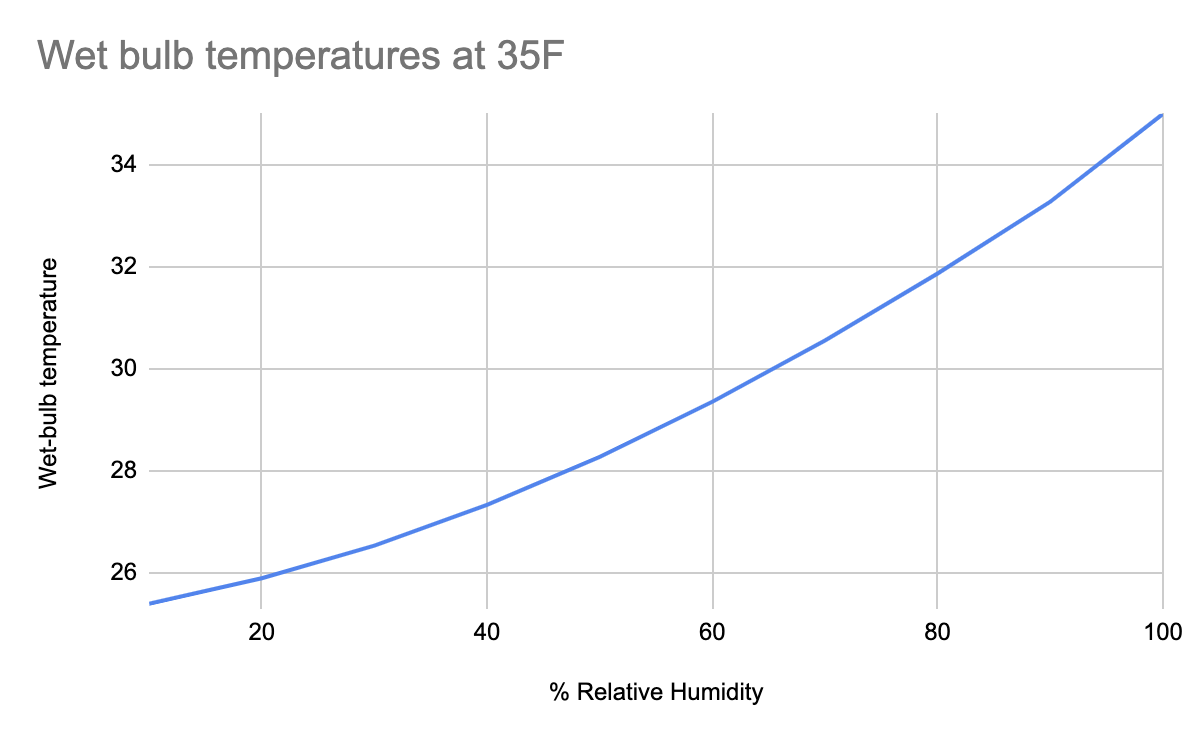
The strange thing about evaporative cooling is that it falls along a logarithmic curve where higher values of relative humidity yield diminishing returns on evaporative cooling. Say the air temperature is 40ºF, and precipitation is forecasted. You want to determine if that precipitation will fall as rain or snow. The relative humidity is initially 80% but falls to 20% after a few hours. The wet-bulb temperature for 80% RH at 40º is 36ºF, which is well above the threshold for snow, which means it will rain. However, with 20% RH, you’re talking about a 29ºF wet-bulb temperature, which could yield snow. The theoretical maximum temperature that could yield snow is about 48ºF, which necessitates a perfect situation with RH at about 5%, which seldom happens in the mountains.
So what are the implications for wet bulb temperature and evaporative cooling in skiing? First, as I mentioned above, it can determine whether rain or snow will fall when the air temperature is above freezing. This becomes especially important in the spring when the difference between rain and snow is just a few degrees. Secondly, evaporative cooling can also produce good freezes for corn harvesting in the springtime. Finally, even if the air temperature is 40ºF, if you have a cold, clear night with low relative humidity, you can still get a really good freeze, which is ideal for springtime backcountry skiing.
- Related: Corn Snow: The Powder of Summer
Finally, evaporative cooling can improve the snow quality during the winter after the snow has fallen. For example, suppose a storm brings relatively dense, heavy snow followed by one or more nights of cold, clear conditions. In that case, evaporative cooling will aid in lowering the temperature of the top layers of snow, drawing moisture out of the snowpack and into the air. This will yield lighter, fluffier snow, which is usually more fun to ski.
Evaporative cooling is a nuanced physics concept that factors into skiing significantly. A basic understanding of it will allow you to make educated decisions about how and where to find the best skiing, which is always the goal!